Human Gene Therapy:深圳先进院陈志英研究组发表非病毒基因载体技术治疗B细胞淋巴瘤研究进展
近日,国际知名杂志《Human Gene Therapy》上发表在线了中国科学院深圳先进技术研究院陈志英研究团队题为“Treatment of human B-cell lymphomas using minicircle DNA vector expressing anti-CD3/CD20 in a mouse model”的研究文章。论文报道该团队的核心技术非病毒基因载体微环DNA表达双靶向抗体治疗人B细胞淋巴瘤。
近年来,癌症免疫治疗有重大突破,若干治疗技术使部分病人病情长期稳定甚至痊愈,表明免疫治疗是人类攻克癌症的希望所在。微环DNA表达双靶向抗体的癌症免疫治疗技术成为继CAR-T技术(“嵌合抗原受体T细胞免疫疗法”)、TIL技术(“肿瘤渗润淋巴细胞技术)、免疫检查点抑制剂等技术之后又一项获得成功的新一代治疗技术。
陈志英团队多年来致力于研究微环DNA表达双靶向抗体的癌症免疫治疗技术。微环DNA是陈志英团队发明的一种非病毒基因载体,因其卓越的基因表达功能,方便的制备技术,已被全球生物医学界广为应用,并在全世界销售。双靶向抗体是一种成熟的抗癌技术,通过链接病人体内的T细胞和癌细胞,达到杀灭癌细胞的目的,其应用比CAR-T和TIL技术方便,FDA已经批准两种双靶向抗体进入市场。但提纯的双靶向抗体半衰期短,需要用微泵连续给药,非常不便,费用高昂。
最近,陈志英团队取得相关进展,是用带有萤火虫荧光基因的人B细胞淋巴瘤细胞株Raji接种到免疫缺陷小鼠,然后将微环DNA(MC.CD20)一次注射到小鼠肝脏,在体内持续表达治疗水平的抗B细胞淋巴瘤(抗-CD3/CD20)的双靶向抗体,并提供人的T细胞。因为Raji细胞在小鼠发出的荧光与癌细胞成正比,用小动物成像仪可以实时监测疗效(如图)。结果标明,同时接受MC.CD20与人T细胞的治疗组小鼠瘤负荷显着低于对照组,生命显着延长,证明抗癌微环DNA治疗癌症的可行性。目前,陈志英团队正在开发把微环DNA注射到肌肉表达抗癌双靶向抗体的技术,一旦成功,便可实现“治疗癌症如打疫苗”一样安全、方便、可负担。微环DNA在肌肉表达抗癌双靶向抗体技术可能为人类攻克癌症做出重要贡献。
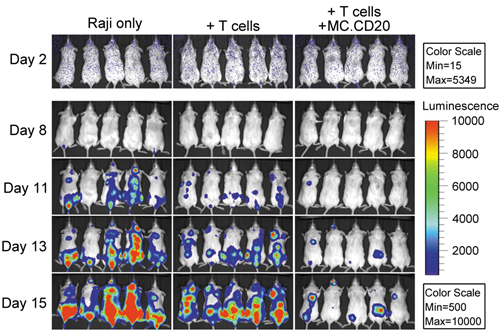 载体技术治疗B细胞淋巴瘤研究进展">B细胞淋巴瘤Raji细胞携带荧光基因,图中治疗组(T Cells+MC.CD20)小鼠荧光强度显着少于对照组(Raji only和T cells),表明微环DNA表达抗-CD3/CD20抗体大大降低瘤负荷,有显着疗效
载体技术治疗B细胞淋巴瘤研究进展">B细胞淋巴瘤Raji细胞携带荧光基因,图中治疗组(T Cells+MC.CD20)小鼠荧光强度显着少于对照组(Raji only和T cells),表明微环DNA表达抗-CD3/CD20抗体大大降低瘤负荷,有显着疗效
原文链接:
Treatment of human B-cell lymphomas using minicircle DNA vector expressing anti-CD3/CD20 in a mouse model.Human Gene Therapy
原文摘要:
Bispecific antibodies (BsAbs), capable of directing T cells to kill specific cancer cells by transiently binding the two cell types, have emerged as one class of promising cancer immunotherapies. However, their wide clinical application might be hampered by two deficiencies: high cost and inconvenience in drug administration. This study presents concept-proving data that these problems could be bypassed by using an enhanced nonviral DNA vector minicircle (MC) to produce BsAb in vivo. It was found that the anti-CD3/CD20 produced from the minicircle (MC.CD20) could effectively mediate the T-cell killing of multiple CD20-positive human B-cell lymphoma cell lines in vitro. More importantly, it was demonstrated that delivery of 5?μg of MC.CD20 to mouse liver via hydrodynamic injection resulted in both the expression of a therapeutic level of anti-CD3/CD20 throughout the 32-day experiment and effective anticancer activity in a B-cell lymphoma xenograft mouse model. The data suggest that MC encoding the BsAbs may become an attractive cancer immunotherapy modality based on its excellent features of safety, efficacy, and convenience in both preparation and use, and its affordability once the delivery technology matures.
相关会议推荐

2017”肿瘤免疫+”研讨会
会议时间:2017.3.10 -3.11 会议地点:上海
会议详情: http://www.bioon.com/z/2017tumor/